Fluorenol has become a point of interest in pharmacology circles over the past decade, especially among readers exploring the broader landscape of wakefulness-promoting compounds. Although it is sometimes mentioned in conversations about agents like Modafinil, fluorenol stands chemically apart. Unlike eugeroics that have undergone formal clinical testing and regulatory scrutiny, fluorenol occupies an entirely different scientific category—one grounded in structural chemistry rather than therapeutic evidence. This article examines ingredients of fluorenol. These ingredients are not additives or fillers as in pharmaceutical formulations, but the atomic building blocks that define how the molecule behaves in biological environments. For a general audience, understanding these basics helps clarify why fluorenol differs dramatically from regulated wakefulness medications and why researchers treat the molecule cautiously.
What Is Fluorenol? A Structural Overview
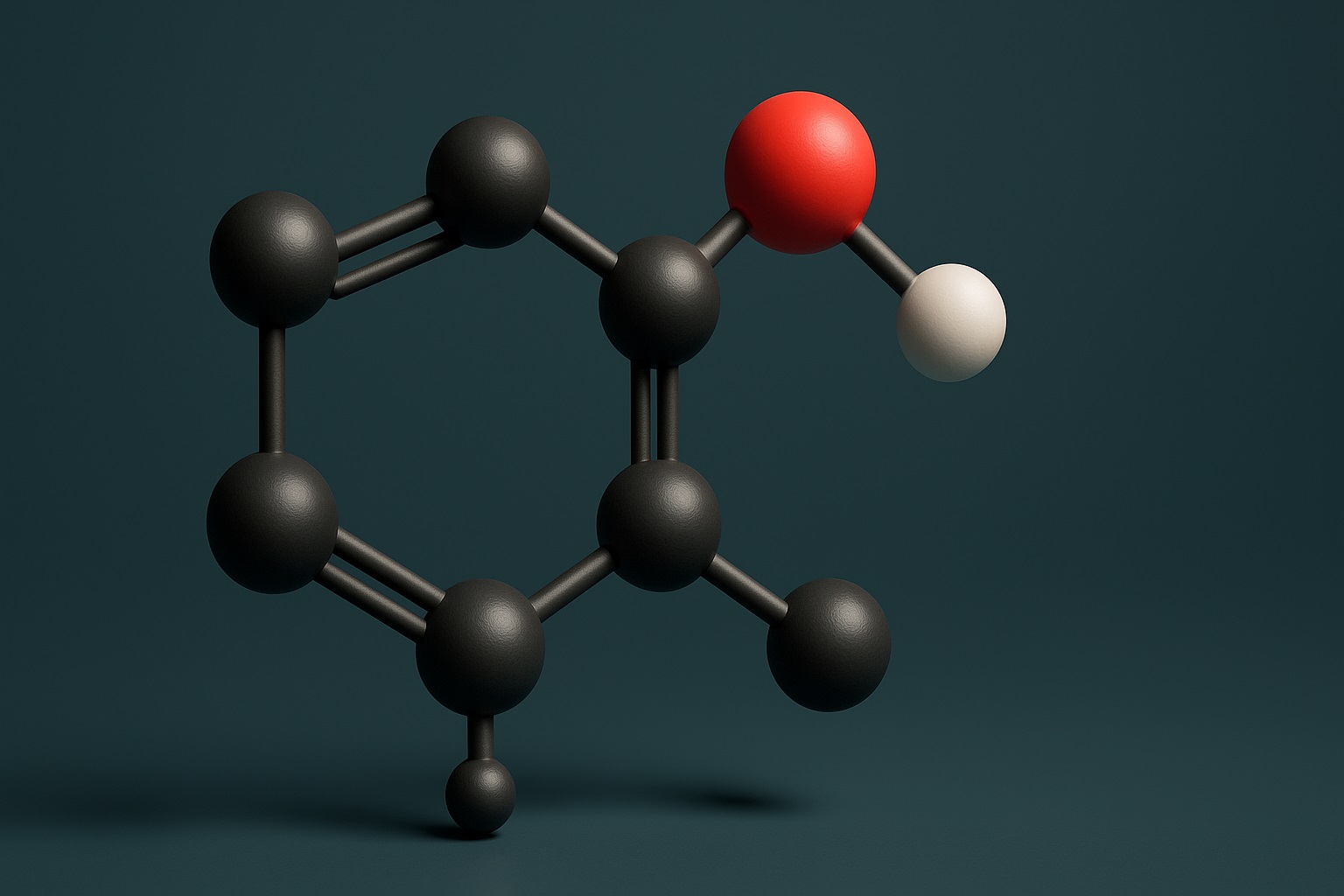
Fluorenol—chemically known as 9-hydroxyfluorene—is a polycyclic aromatic alcohol composed of:
- A fluorene ring system (two benzene rings fused to a five-membered cyclopentane)
- A single hydroxyl group (-OH) at the 9-position
- A compact, rigid carbon backbone
This structure gives the compound its aromatic, hydrophobic character. Because of this architecture, fluorenol can dissolve into lipids more readily than water, influencing how it moves through biological membranes.
Fluorenol is occasionally discussed alongside structurally unrelated compounds such as Fluorenol and Flmodafinil. However, these comparisons are often superficial—fluorenol is not a pharmaceutical, not a supplement, and not a proven eugeroic. Its relevance is primarily scientific rather than clinical.
Chemical Ingredients of Fluorenol
Although fluorenol is a single molecule and not a formulated product, its “ingredients” can be understood as the functional chemical components that give rise to its behaviour.
1. Carbon Framework (C₁₃ Aromatic Backbone)
The molecule contains thirteen carbon atoms arranged in two fused benzene rings and a cyclopentane bridge. This rigid aromatic framework influences:
- chemical stability
- lipid solubility
- membrane permeability
- metabolic transformation pathways
Aromatic hydrocarbons frequently bind to hydrophobic receptor pockets and have predictable metabolic outcomes, including oxidation and conjugation.
2. Hydrogen Skeleton (H₁₀ Distribution)
Hydrogen atoms saturate the carbon framework, reinforcing the hydrophobic nature of the molecule. Hydrophobic compounds often:
- penetrate the blood–brain barrier efficiently,
- persist within lipid-rich tissues,
- exhibit prolonged or unpredictable elimination kinetics.
This contrasts sharply with the pharmacokinetic profiles of clinically validated agents such as Armodafinil, whose stereochemistry and hydrophilicity patterns are documented in FDA clinical labeling data (FDA.gov).
3. Hydroxyl Group (-OH)
The single hydroxyl group is the molecule’s most pharmacologically relevant feature. It:
- increases polarity at one site,
- allows hydrogen bonding with proteins,
- facilitates Phase II metabolism (glucuronidation),
- slightly improves water solubility over fluorene.
This functional group strongly influences how the molecule is processed by the liver.
Why “Ingredients” Matter in Clinical and Scientific Settings
In medical pharmacology, the composition of a molecule determines its therapeutic potential, safety profile, and metabolic fate. Clinicians routinely examine:
- lipophilicity
- functional groups
- predicted metabolites
- ability to cross biological barriers
Fluorenol’s aromatic hydrocarbon foundation raises questions familiar to toxicologists. Polycyclic aromatic compounds can yield reactive intermediates under hepatic metabolism—one reason fluorenol has never been evaluated as a drug candidate in FDA, MHRA, or TGA submissions.
Fluorenol vs. Known Eugeroics
Fluorenol is not structurally similar to:
- Adrafinil
- modafinil-derivatives
- histaminergic agents
- dopamine/norepinephrine reuptake inhibitors
Even though its name occasionally appears near discussions of wakefulness agents, fluorenol is not included in the official Eugeroic Drug List, nor is it regulated as a therapeutic compound.